
Insights+: The US FDA New Drug Approvals in February 2024
Shots:
-
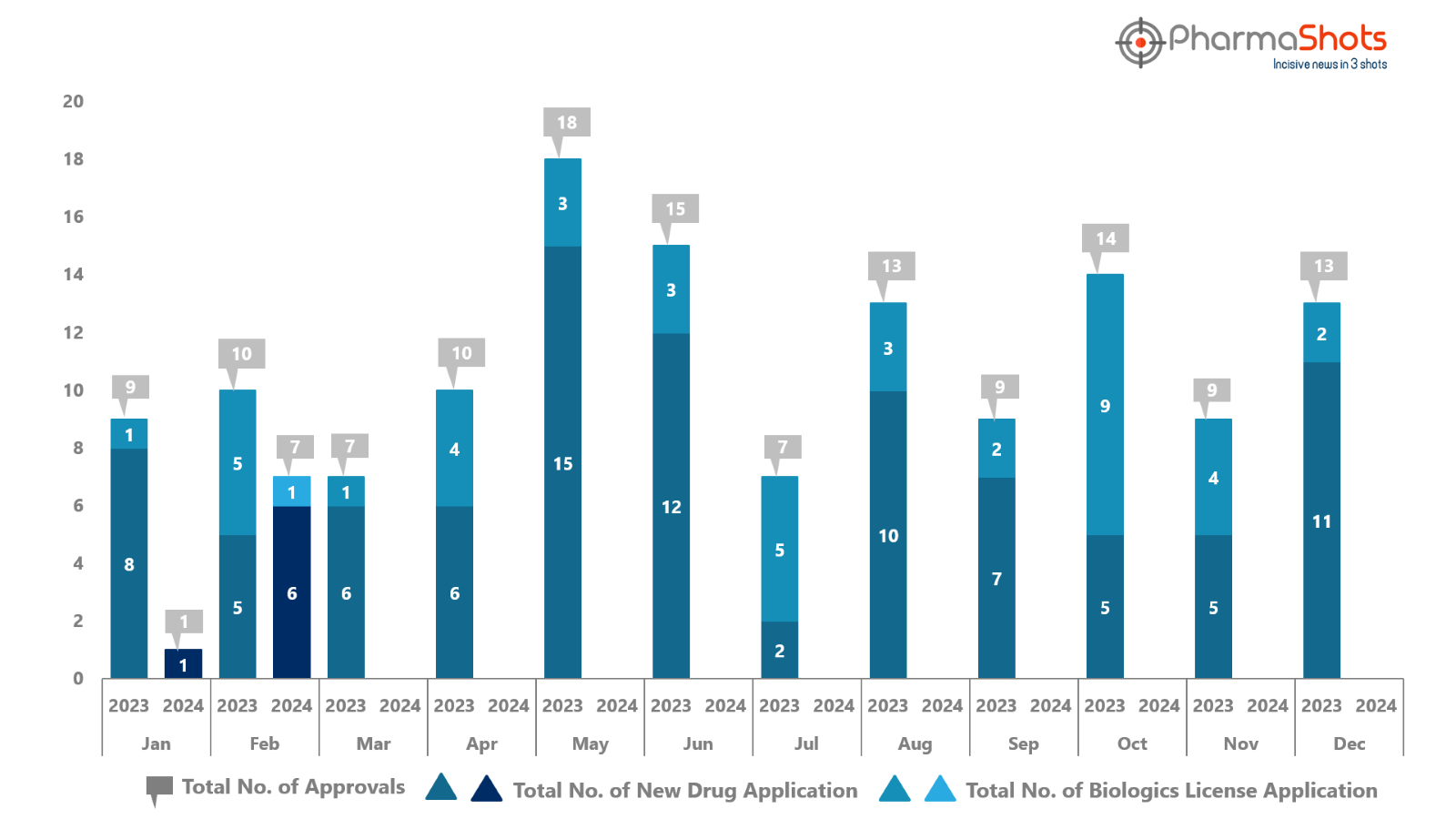
PharmaShots has compiled a list of US FDA-approved drugs in the month of February 2024
-
The US FDA approved a total of 7 drugs including 6 new molecular entities and 1 biologic leading to the treatments for patients and advances in the healthcare industry
-
The major highlighted drug was Takeda’s Eohilia (budesonide oral suspension) for treating eosinophilic esophagitis (EoE)
Active Ingredient: Budesonide
Approved: Feb 09, 2024
Company: Takeda
Disease: Eosinophilic Esophagitis
-
The approval was based on the results from 2 trials (Study 1) & (Study 2) evaluating the safety & efficacy of Eohilia (2mg, BID) vs PBO in patients (aged 11-56yrs. & 11-42yrs.) with EoE. The efficacy endpoints of the trials were histologic remission & change from baseline in patient reported DSQ post 12wks of treatment
-
The results depicted that 53.1% vs 1% & 38% vs 2.4% of patients achieved histologic remission whereas the absolute change from baseline in DSQ combined score were -10.2 vs -6.5 & -14.5 vs -5.9. It was also seen that Eohilia was not safe & effective as EoE treatment for longer than 12wks.
-
Eohilia (corticosteroid) expected to be made available in 2mg/10mL dosage by the end of Feb 2024
Active Ingredient: Iloprost
Approved: Feb 13, 2024
Company: Eicos Sciences
Disease: Severe Frostbite
-
The approval was based on the study evaluating the efficacy of Aurlumyn for treating patients (n=47) with severe frostbite, all received aspirin & SoC. Patients were divided into 3 treatment arms receiving Aurlumyn (IV, 6hrs., daily for up to 8 days) alone in arm 1 & unapproved medications with/without Aurlumyn in arms 2 & 3
-
The 1EP was a bone scan taken 7 days post initial frostbite for predicting the need for amputation of at least 1 finger or toe
-
The results, on day 7, demonstrated the need for amputation in 0% of patients across arm 1 vs 19% & 60% of them in arms 2 & 3 respectively with a lesser presence of the bone scan abnormality in the groups receiving Aurlumyn
Active Ingredient: Cefepime Hydrochloride; Enmetazobactam
Approved: Feb 22, 2024
Company: Allecra Therapeutics
Disease: Complicated Urinary Tract Infections incl. Pyelonephritis
-
The US FDA has granted approval to the company’s Exblifep for the treatment of complicated urinary tract infections (incl. pyelonephritis) patients (18yrs. & above) along with a 5-yr. marketing exclusivity extension until 2032
-
The approval was based on clinical data that showed the effectiveness of Exblifep against antimicrobial resistance in gram –ve bacteria, especially resistance mediated by both ESBL and AmpC and data from the P-III (ALLIUM) study which met the 1EP of non-inferiority and superiority with the drug vs piperacillin/tazobactam
-
Shanghai Haini Pharmaceutical is the drug’s exclusive license holder across Greater China and Advanz Pharma in the EU region
4. The US FDA Approves Hugel’s Letybo for the Treatment of Glabellar Lines
Active Ingredient: Letibotulinumtoxina-WLBG
Approved: Feb 29, 2024
Company: Hugel
Disease: Glabellar Lines
-
The US FDA has granted approval to the company’s Letybo for 50 and 100 units to treat moderate-to-severe glabellar lines in adults
-
The approval was based on the data from three P-III completed studies that recruited more than 1,000 participants across the US and the EU
-
The data from the clinical studies showed the effectiveness and favorable safety profile of Letybo as a treatment for glabellar lines
Note:
According to the FDA's February 2024 approval list, Legubeti (Feb 13, 2024), Pantoprazole Sodium in 0.9% Sodium Chloride (Feb 14, 2024) and Sehippy (tentatively approved on Feb 16, 2024) were also approved; however, no PR was available
Related Post: Insights+: The US FDA New Drug Approvals in January 2024
Tags

Disha was a content writer at PharmaShots. She is passionate and curious about recent updates and developments in MedTech and Pharma industry. She covers news related to clinical trial results and updates. She can be contacted at connect@pharmashots.com.














